You are here
Publications & Research
Publications & Research

Publications & Research
The HKJCDPRI Publications Section contains collaborative researches and publications with our partners and renowned academic institutions, and other research and development projects related to disaster preparedness and response.
The Guidelines section contains our selected collection of technical information, operational guidelines and useful tools for disaster management.
The Blog sub-section provides a platform where our team and peers share news and updates, as well as opinions and experiences in building disaster preparedness for the communities.
The blog posts are written by the author in his own personal capacity / affiliation stated. The views, thoughts and opinions expressed in the post belong solely to the author and does not necessarily represent those of Hong Kong Jockey Club Disaster Preparedness and Response Institute.
All resources listed here are freely and publicly available, unless specified otherwise. We ask users to use them with respect and credit the authors as appropriate.
2020
Dr. Jimmy Chan
With thousands of micro-organisms -such as bacteria and viruses in the environment, we are all vulnerable to infection. The skin and mucous membrane are the first barriers to protect us from the invasion of these micro-organisms. If the microbes successfully colonize through these broken barriers, our body will initiate an inflammatory response (red, swelling, pain and heat in that area) to counteract the invasion. Blood flow will increase in that region and will bring white blood cells and macrophages to clear up the invaders. However, if this mechanism fails, our body immune system will be stimulated to generate high power defense against these micro-organisms.
With a spike on its surface (Fig. 1) the COVID-19 virus can tap into the ACE2 receptors of human cells, simulating using a key to open the lock of a door. This protein is an antigen that can trigger the immune response in our body to produce humoral and cellular response specific to COVID-19 virus. The humoral response will produce antibody around one week’s time to kill the virus (like a missile) whereas the cellular response will take about 1-2 weeks to produce cytotoxic T-cells to destroy the virus (like a sniper chasing to kill the target). The antibody and cytotoxic T-cell level of a patient in recovery phase will drop as time goes by. If second infection occurs because of immune memory, the antibody and cytotoxic T-cell can be built up quickly to combat against the virus.

Figure 1: S-Protein of COVID-19 Virus (From Internet: CDC/US)
After recovering from the infection of COVID-19 virus, the patient will be protected by the body immune system. Vaccination is another way of receiving immune protection. By injecting harmless COVID-19 antigen into our body, it can help the immune system develop protection from the disease. Vaccines are biological products that prevent and control the occurrence of epidemic infectious disease. The development of vaccine follows a standard procedure with pre-clinical and clinical stages. In pre-clinical stage, the vaccine type will be decided. The antigen is isolated from the virus. After purification, the vaccine will be tested in animal model to ensure safety and adequate effectiveness of the immune response. If the pre-clinical phase is successful, then they will proceed to test the vaccine in clinical stage.
In the clinical stage, there are 4 phases:
- Phase 1 clinical trial: This is a small scale trial and focus on vaccine safety and effectiveness of the immune response.
- Phase 2 clinical trial: This is a larger scale clinical trial. In addition to immune effectiveness and safety, it also checks the vaccine optimal dosage, single or multiple shots and the side effect of the vaccine in a controlled environment.
- Phase 3 clinical trial: This is a very large-scale clinical trial which usually involves more than 10,000 volunteers. It will check the parameters as stated in phase 2 clinical trial, but it is under a natural environment.
- Phase 4 clinical trial: If the vaccine can pass the phase 3 clinical trial, then a formal preparation will be submitted and get approval from appropriate authority for the clinical use of the vaccine. Post-marketing surveillance will be monitored for a prolonged period of time.
Different types of COVID-19 vaccines will be available:
- Live attenuated vaccine: the virus is made less virulent but the antigen stimulation power is preserved.
- Inactivated vaccine: the virus is killed and the antigen stimulation power is preserved.
- Adenovirus vaccine: The adenovirus is a common respiratory tract virus. It is made less virulent and is implanted with the COVID-19 genetic material. The adenovirus serves as a vehicle to transport the COVID-19 antigen into our body to stimulate the immune response.
- Recombinant vaccine: Recombinant vaccines are made by using bacterial or yeast cells to manufacture the vaccine.
- Messenger RNA (m-RNA) vaccine: mRNA can serve as a messenger to direct the body cell to produce antigen. The genetic material of COVID-19 antigen is incorporated into the mRNA and act as a vaccine to stimulate the immune response. The advantages of mRNA are shorter clinical trial period and cheaper to produce.
Up till now, more than 300 COVID-19 vaccines are being developed in many countries. Many of them are in phase 3 clinical trials. Nevertheless, there are several outstanding vaccine developments in China, United Kingdom and United States (Fig. 2). According to World Health Organization (as at 2 October, 2020), there are 4 vaccines in phase 3 clinical trials in China (Sinovac Biotech, CanSino Biologics and China National Biote Group). Inactivated and Adenovirus vaccine types are probably adopted. Since the control of COVID-19 infection in China is good, with minimal new cases, the phase 3 clinical trial is conducted in other epidemic areas like Middle East and South America countries. According to the National Health Commission and news reports, the immune protection and side effects results are very encouraging.
Figure 2: COVID-19 vaccine development
(Internet photos from Xin Hua News, Belt and Road News and South China Morning Post)
In United Kingdom, researchers produced the Oxford Vaccine (AstraZeneca) with the SARS vaccine technology. The adenovirus is used as vehicle to transport the antigen into our body to stimulate the immune response, claiming that they can provide double protection on both the humoral and the cellular immune response. However, a very serious side effect (Transverse Myelitis leading to paralysis) has been detected in the phase 3 clinical trial recently. According to the information in AstraZeneca website on 2 October 2020, a standard review process triggered a voluntary pause to vaccination across all global trials on 6 September to allow review of safety data by an independent committee. Their recommendations have been supported by international regulators in the UK, Brazil, South Africa, India and now in Japan, who have deemed that the trials are safe to resume. However, The FDA of US has not made a decision yet.
In United States, researchers produced the mRNA vaccine (Moderna and Pfizer). This is a brand new technology with no historical records. Published in New England Journal of Medicine, the study is now also in phase 3 clinical trial and two vaccine doses are required to be injected. The side effect in first dose is mild but more prominent in second shot. The immune response is good and the antibody level is similar to that of a recovery COVID-19 patient.
The following factors should be considered before deciding the type of vaccine taken:
- Affordable cost
- The level of protection the vaccine provided such as complete immunity to reduce mortality and morbidity.
- The duration of protection.
- Safety, side effect and complications of the vaccine.
- Degree of protection with respect to the mutation of the COVID-19 virus.
It is expected that the demand for COVID-19 vaccine will be great. Those who face the greatest risk such as the emergency personnel, including medical/nursing staff, disciplinary forces and government decision makers should have the first priority to receive vaccination. The second tier should be the high-risk groups such as old age home residents, food handlers and people that need to work in crowded environment. The last tier should be common people.
Dr. Jimmy Chan,
President of HK Association for Conflict and Catastrophe Medicine.
FHKAM(Surgery), FHKAM (Emergency Medicine)
Regional Director (HK), Advanced HazMat Life Support International, USA.
Reference:
1. Vaccines and immunization, World Health Organization (WHO)
2. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial, The Lancet, 15 August 2020
3. New study reveals Oxford coronavirus vaccine produces strong immune response, The University of Oxford, 20 July 2020
4. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report, The New England Journal of Medicine, 14 July 2020
5. Pfizer and BioNTech Propose Expansion of Pivotal COVID-19 Vaccine Trial, Pfizer, 12 September 2020
6. Types of vaccine, Vaccine Knowledge Project, The University of Oxford, 3 January 2019
7. COVID-19 vaccine AZD1222 clinical trial resumed in Japan, follows restart of trials in the UK, Brazil, South Africa and India, AstraZeneca, 2 October 2020

[This article is only available in Chinese.]
[This article is only available in Chinese.]
每年10月13日為聯合國的「國際減災日」,目的為提高世界各國對防災減災的意識,並藉此鼓勵各國在《2015-2030仙台減災綱領》(Sendai Framework of Disaster Risk Reduction)下,邁向減低因災害而造成全球損失的目標。
今年國際減災日的主題為Disaster Risk Governance,意即呼籲各國繼續提高減災的「治理能力」。聯合國秘書長減災事務特別代表(Special Representative of the Secretary-General for Disaster Risk Reduction and Head of UNDRR)水鳥真美(Mami Mizutori)在介紹今年國際減災日主題時,指出良好的治理包括清晰的(減災)遠景、執行計劃、以科學為行動依據、有能力及被賦予相稱權力及職能的機構。 [1]
「治理」這個議題可能對大多數人來說太遙遠。然而用心想想,當今年人類面對COVID-19--- 一種比颱風地震造成更深遠影響的人畜共通傳染病(Zoonotic Diseases)時,我們其實也對災害治理有了更深的體會。
防疫工作的有效性,關鍵在於政府對新病毒的態度、決策部門與科學及醫療界別的合作、保障公共衛生而立法及實施的適時性及相關性、政府與民間(包括非政府組織)的互信及協調的有效性等。疫情的控制反映減災治理是否有效,而且效果是顯而易見的,經歷過COVID-19,相信社會及個人都對所在地區的減災治理能夠有深入的分析及看法。
事實上,監督政府在防災減災上是否發揮有效管治,無論社區或個人都有共同責任。在「後COVID時代」,氣候變化和全球經濟衰退的大環境下,各類災害的風險只會越來越高。因此,就讓我們繼續承擔一個21世紀公民應有的責任,監督政府及其他參與單位在減災治理上的作為,使我們的社區及國家承擔減少因災害帶來破壞的全球目標,並作出應有的貢獻。
希望今年10月13日聯合國的「國際減災日」,對我們都有新的啟發及感受。
參考資料:
[1] COVID-19 and the climate emergency tell us all we need to know about disaster risk governance, Press Release, UNDRR, 4 September 2020 (只提供英文版)
筆者簡介

林鈞浩 Kwan-Ho Timothy LAM
世界主義、人道主義者、曾在逾五十個國家生活及工作。
香港賽馬會災難防護應變教研中心 Technical Expert for Professional Development 。
英國約克大學 Post-War Recovery Studies (戰後重建) 碩士畢業,十多年從事人道事務工作。2018-19年為國際紅十字會 (IFRC) 美洲加勒比海辦事處主管。

[This article is only available in Chinese.]
[This article is only available in Chinese.]
2020年已經過了一大半,眨眼間來到九月份,相信大家早已適應生活「新常態」。除了忙於日常抗疫之外,你留意到2020年的天氣曾經出現異常嗎 ?
2020上半年天氣回顧
根據天文台公布的每月天氣回顧,本港在六月至八月期間經歷了有記錄以來最熱的夏季 [1][2] 。而且,本年八月的酷熱天氣日數為16天,是有記錄以來八月份最多的日數。
除了氣溫破新高之外,6月份還刷新了幾項「新紀錄」,包括天文台於6月6日早上發出自2017年5月以來的首個黑色暴雨警告信號,當天亦錄得超過14,350次雲對地閃電,是自2005年推出閃電定位系統以來的第二高紀錄;此外,6月8日早上在機場附近亦有水龍捲報告。
雖然2020年上半年的累積雨量為963.4毫米,較同期正常值1096.9毫米少約12%。可是,某些地區(如西貢、大澳、鯉魚門等)的水浸情況卻愈趨嚴重。以西貢為例,北圍村、蠔涌以及慶徑石村均有出現嚴重水浸,令居民損失慘重。
教研中心一向致力提升脆弱社區的應災韌性,中心團隊早前特別到西貢鄉郊及鯉魚門等受災風險較高的地區視察,並與社區關鍵持份者會面,了解該社區正在面對的災害風險、地理獨特性及現時實行的應災方案,並辨別合適的社區持份者,以攜手推動「防災社區」的發展,讓居民可以於下次颱風和暴雨來臨之前能夠防患於未然,減低人命傷亡及財物損失。
何謂「防災社區」?
一個理想的防災社區應該結合社區的獨特性,並加入災害管理四大階段的能力—「備災」、「應變」、「恢復」及「滅災」。要達致「防災社區」,需要由一個牽頭組織透過社區賦權(community empowerment)的過程來凝聚社區成員的共識與力量,建立社區專屬的防災網絡,當中包括政府組織、社福機構、防災機構,更可以邀請區議員、商戶及業主立案委員會加入,構建鄰里之間的防災社區氛圍,讓社區在緊急情況下都能夠「自救」。
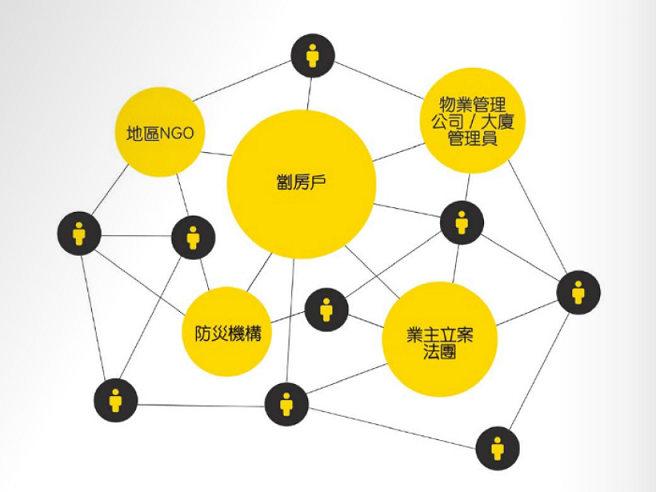
圖一︰「社區互助網絡」模式 [3]
教研中心與及香港聖公會麥理浩夫人中心團體及社區工作部共同策劃『「無惘。知災」舊區居民社區教育計劃』,於葵青區初步建立防災社區網絡加強劏房戶的防災及應災能力。以上圖表顯示該防災社區網絡包括的社區成員。
「防災社區」藉由推動防災和減災的措施,如設立各單位在應災時的角色、規劃疏散路線、加強巡視容易發生災害的地點、進行防災演練等,以減少社區的易致災因子,從而降低災害發生的機會;而當萬一災害發生時,「防災社區」亦能防止災情的擴大、降低災害的損失,並能迅速推動復原和重建工作。
推動防災社區不但可以減少災害發生的機率及降低災害造成的損失,亦可以藉此提升社區的危機意識,共同營造安全的生活環境。

圖二︰2017年超強颱風「天鴿」襲港,屬低窪地帶的鯉魚門成為重災區。
圖片來源:On.cc (報導連結:連結)

圖三︰洪水湧入鯉魚門街道,消防員站在一旁暫避。
圖片來源:蘋果日報 (報導連結:連結)


圖四、五︰政府部門於超強颱風「天鴿」之後,分別在鯉魚門岸邊加建防波堤,並在行人路邊築起1米高石屎牆,阻止海浪湧上岸,兩者在「山竹」襲港時均發揮功效。
圖片:教研中心攝

圖六︰西貢蠔涌村附近有一居所位於濠涌河旁,如遇上水漲,河水有機會沖入屋內,造成傷亡或財物損失。
圖片:教研中心攝

圖七︰西貢對面海新村,在2018年超強颱風「山竹」吹襲過後,有一艘白色小船被吹至岸上,至今仍未清理。
圖片:教研中心攝
參考資料:
1. 二零二零年六月天氣回顧 ,香港天文台, 2020年7月
2. 二零二零年八月天氣回顧,香港天文台, 2020年9月
3. 有關風災對不適切居所居民影響問卷調查報告, 香港聖公會麥理浩夫人中心, 2020年2月
由香港賽馬會災難防護應變教研中心經理(社區協作)陳靜怡撰寫
延伸閱讀︰
「有關風災對不適切居所居民影響調查」發佈會已於5月24日順利舉行

[This article is only available in Chinese.]
[This article is only available in Chinese.]
這個標題是騙你進來學點化學知識的。
我曾「秘密地」受一位傳媒朋友所托,用滴定 (titration) 方法分析了幾個牌子的次氯酸水產品。它們價錢由每樽數十元至三、四百元不等,那它們的有效成份 — 次氯酸濃度又有多大分別呢?
次氯酸係咩嚟?
次氯酸 (英文:hypochlorous acid),化學式為HClO (分子結構見下圖)。其實次氯酸和漂白水中的「次氯酸鈉」(NaClO) 只差一個鈉離子,次氯酸水是種不穩定弱酸,又怕熱又容易被紫外線分解,失去消毒功效。而漂白水是強鹼性,次氯酸鈉溶液比較穩定。而兩者的消毒效能都是因為次氯酸根離子 (ClO-) 這個強氧化劑,將病毒、細菌結構氧化和破壞。
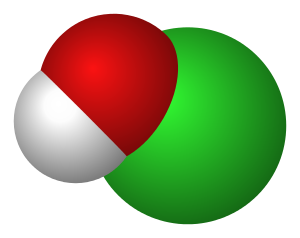
在泳池會聞到輕微刺鼻的「氯氣味」。將氯氣通入水中,會形成鹽酸和次氯酸。但是氯氣本身有毒又不易控制,所以消毒泳池水時其實不是用氯氣,而是在水中加入強鹼性的工業用漂白水,然後再加酸調教泳池水pH值回到中性。
市面上買到的次氯酸水,也就是同樣的製造原理。
說回我的「秘密」檢測也實非什麼秘密任務,只是該偵查報道搜集的題材太豐富,而節目時間卻太短,一個「咩Q水」已經太爆,因此我的訪問部份只出了三分鐘。不夠時間出的次氯酸水檢測結果,在這裡分享少少給大家看看:
| 產品A | 產品B | 產品C | 產品D | |
| 每100毫升售價位# | $44.8 | $69.8 | $85 | $120 |
| 次氯酸濃度* | 188ppm | 103ppm | 35ppm | 181ppm |
#於2020年8月29日網上搜尋產品的本地零售價(港元)。
*滴定反應方法參考自2020年3月16日科大陳鈞傑教授於《上網問功課》節目的示範。URL: https://youtu.be/GNGppgqLPVQ?t=1767
看見某牌子標榜「天然白細胞殺菌因子」時我笑了。其實也算是對的,上生物堂學過,身體內嗜中性白血球 (Neutrophil) 能夠產生次氯酸來殺滅細菌。如果你曾經因為「天然」而豪氣花錢買這產品後自覺笨了,現在會否想跟化學KOL自家調配次氯酸水?
市面上賣的次氯酸水太貴不如自己溝?
理論上是可以的,材料只有水、漂白水,和廚房找到的白醋。只要各材料比例正確,就能溝出與市面上買到濃度相約的次氯酸水。
首先要注意安全,稀釋前的家用漂白水次氯酸鈉標準濃度約5.2% (即52000ppm),強鹼性(pH~14)會刺激皮膚,操作時需要戴手套,並在空氣流通的地方進行。
自家製次氯酸水成本有幾低?
第二就是計清楚條數,假設一樽1500亳升的家用漂白水為16元,一樽470毫升食用白醋為10元,而水費真的十分平,暫且當它零成本。如要調配1000毫升,濃度100ppm的次氯酸水:
//先準備1000毫升清水,加入2毫升家用漂白水,搖勻。再加入5毫升白醋,令溶液pH降至5.5 - 6.0,再搖勻便完成。//
成本還不用2毫子!
但是,更須注意的是不同牌子的漂白水次氯酸鈉含量相差可以很大,參考2009年的消委會《選擇月刊》報告,次氯酸鈉濃度由2%至6%不等,相差竟可達3倍!至於食用醋亦不可跟工業用醋混為一談,說來說去又是濃度和雜質問提。家中沒有pH計可即時量度溶液pH值(較易買到的pH試紙不能用,因為漂白水會「漂白」試紙中的指示染料啊!),若果醋加多了,過酸的環境會令毒氣釋出,到時不能殺菌更反而增加危害風險。
還有,不論是次氯酸或次氯酸鈉,濃度過高都會對人體有害。因此,對於怕麻煩又怕計錯數溝錯漂白水的市民,以下最基本的清潔/消毒用品會較適合。
三個平靚正方法消滅新型冠狀病毒(SARS-CoV-2):
- 用肥皂或梘液洗手最少20秒,肥皂分子滲入病毒表面的脂肪結構,原理就如洗潔精瓦解油污,然後用水沖走。普通梘液已經有效,跟有添加「消毒」成份的梘液沒有明顯分別。
- 酒精搓手液、乙醇或丙醇 (60 - 70%) 能溶解病毒脂肪結構和破壞蛋白質結構。酒精搓手液是在沒有梘液或水洗手時的選擇,想清潔一般物件表面,酒精則較漂白水貴了一些。
- 稀釋漂白水^適合清潔物件和地板表面。漂白水中次氯酸鈉的強氧化作用可破壞病毒的蛋白質和核酸結構。清潔需要最少要10分鐘接觸時間,然後過水或抹乾。注意漂白水屬鹼性會刺激皮膚,因此不應用來洗手。亦切勿將漂白水混合其他清潔劑,否則可能會釋出毒氣。
日常家居清潔是必須,在人多或空氣不流通的地方戴好口罩,才是預防新型肺炎的最重要習慣。
^ 市面不同牌子的家用漂白水次氯酸鈉濃度不一,市民必須先看清楚產品使用說明。
麥嘉慧
科學人








