You are here
COVID-19 Tests in Hong Kong
COVID-19 Tests in Hong Kong
Dr. Jimmy CHAN
From 1 September 2020, the Hong Kong Government started to offer free Universal Community Testing Program of COVID-19 for Hong Kong citizens. In this blog, we shall discuss different kinds of COVID-19 tests available and the pros and cons of these tests.
1. Viral culture of COVID-19 Test
The viral sample is obtained from the source and then put into a culture medium. If there is positive replication of viral particles in the medium, the result is positive. It indicates that the source is infected or contaminated with live viral particles with potential source of infection. However, this test needs a few days to a week to have the result. Therefore, it is not an efficient approach for prompt diagnosis and measure to stop the viral transmission in the community.
2. PCR COVID-19 RNA Test
The viral sample is obtained from the source and laboratory compares the genetic material obtained with the genetic code of the COVID-19. Since the sample viral concentration may be low, the laboratory will use the primer reagent to enhance the concentration of the genetic material in order to increase the sensitivity of the test. A positive test indicates the presence of the COVID-19 genetic code from the source. However, the sample obtained can be live or dead viral particles. Therefore, this test may not indicate a current infection as the patient may carry residual viral fragments in the recovery phase. The result will be obtained in one to several days. The sensitivity and specificity of this test are high as it is regarded as the golden standard in Hong Kong with an accuracy of around 97%.
3. COVID-19 antibody Test
This is a blood test that checks the presence of the COVID-19 antibodies in our body. After infected by COVID-19 virus, our body immune system will start the immune response to produce antibodies against the virus. There are two kinds of antibodies, namely IgM and IgG. The presence of IgM antibodies suggests recent infection whereas IgG antibodies suggest past infection and the host may be protected from infection. However, the host needs about one week’s time to develop antibodies, therefore, this is not efficient for promptly identifying the viral carriers.
4. Rapid Tests
Two kinds of rapid tests are available. The first one is a finger prick test (the kit is similar to the commercial pregnancy test kit). It can test the presence of IgG or IgM by putting a drop of blood into the test kit. The result can be obtained in about 10-15 minutes. However, this test is neither validated nor standardized, therefore, the false positive or negative rates are quite high.
Harvard Medical School and University of Colorado Boulder suggested another rapid test. This is also a RNA nucleic acid strip test and the result can be obtained in less than 30 minutes. The sensitivity of this test is not as high as PCR test. However, the cost for this test is cheap (about HK$10 for each test) and therefore it may be a good surveillance test for the community, especially the asymptomatic invisible COVID-19 carriers.
It is quite difficult for a virus to enter into the host cell for replication. The COVID-19 virus has a Spike (Figure 1: S-protein of COVID-19 virus) on its surface that can tap into the ACE2 receptors of the respiratory and gut epithelial cells, simulating a key to open the lock of a door. This is the reason why we can get higher yield of the viral particles in the naso-pharynx and the stool. The accuracy of the RNA nucleic acid test depends on the sample collection method. The viral sample can be obtained from nasal cavity, naso-pharynx, oro-pharynx and the deep saliva from the throat (Figure 2). The accuracy is high if the sample is directly taken from the naso-pharynx (70% or higher), but patients may find it uncomfortable when taking the test as it may induce a sneezing or coughing reflex, posing an infection control issue when taking the swab. The combined nasal cavity + oro-pharynx swab is quite accurate and the irritations to the patients are minimal. Therefore, this sampling method was adopted by the free Universal Community Testing Program in Hong Kong.
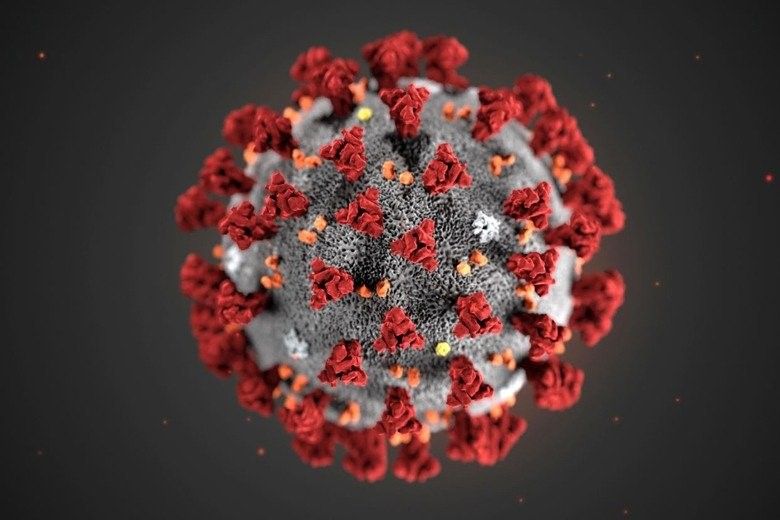
Figure 1: S-Protein of COVID-19 Virus (Source: CDC/US)
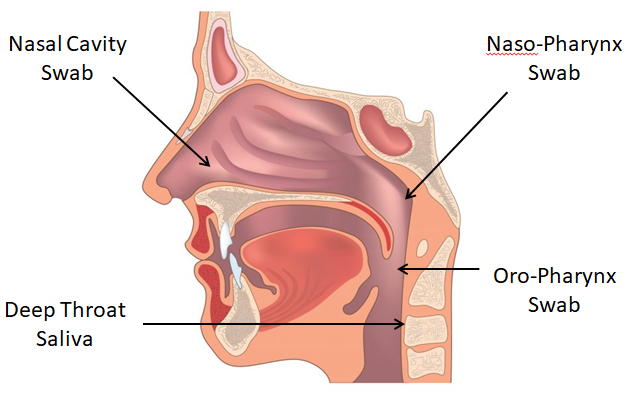
Figure 2: COVID-19 swab taken from different sites in pharynx (Internet free photo)
Lastly, it is important to note that before taking the swab samples, no antiseptic materials like alcohol, mouthwash or gargle should be taken. Otherwise, the result will be affected. Although the surveillance COVID-19 test may not be as accurate as the formal PCR test, however, it is cheap, and the turnaround time is much shorter. A positive surveillance test will be checked against a proper PCR test before confirmation. Therefore, it is a good option to carry out mass screening program to identify the invisible COVID-19 carriers. The Hong Kong citizens should support this program in order to break the COVID infection chain in Hong Kong.
Dr. Jimmy Chan
President of HK Association for Conflict and Catastrophe Medicine.
FHKAM(Surgery), FHKAM (Emergency Medicine)
Regional Director (HK), Advanced HazMat Life Support International, USA.
Reference:
1. HKSAR Universal Community Testing Program for COVID-19 for Hong Kong citizens, Hong Kong Government, 2020
2. Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 surveillance. Preprint. medRxiv. 2020;2020.06.22.20136309. Published 2020 Jun 27. doi:10.1101/2020.06.22.20136309
3. Comparative accuracy of oropharyngeal and nasopharyngeal swabs for diagnosis of COVID-19, The Centre for Evidence-Based Medicine (CEBM), 26 March 2020
4. Vlek ALM, Wesselius TS, Achterberg R, Thijsen SFT. Combined throat/nasal swab sampling for SARS-CoV-2 is equivalent to nasopharyngeal sampling [published online ahead of print, 2020 Jul 14]. Eur J Clin Microbiol Infect Dis. 2020;1-3. doi:10.1007/s10096-020-03972-y
5. COVID-19 Tests in Hong Kong: Where to Go? (UPDATED), 31 August, 2020, AD Medilink